PLAQUE REMOVAL EFFICACY OF A NOVEL ORAL CARE DEVICE: A MICROBIOLOGICAL ASSESSMENT
Inserito il 23/01/2014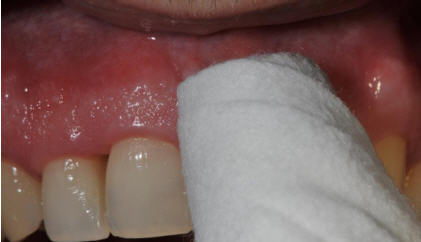
- Dr.ssa Marisa Roncati* - Dr.ssa Alessandra Lucchese**
- * School for Dental Hygienists, Polytechnic of Marche University, Italy. **Department of Medical-Surgical Sciences of Communication and Behavior, Dental School, Ferrara University, Italy.
- Email: marisa.roncatiparma@unibo.it
- Sito Web: www.studioparmabenfenati.it
Figure 1. Clinical use of a novel oral care device for plaque removal.
Full Length Research Paper
______________________________________________________________________
Full Length Research Paper
______________________________________________________________________
Routine toothbrushing is the principal method used by individuals to remove biofilm and control plaque-related diseases, such as periodontitis and caries (Creeth et al., 2009; Lucchese et al., 2012a). However, in some adults, especially those with inflammatory problems, self-performed mechanical plaque removal is insufficiently effective and should be improved (van der Weijden and Hioe, 2005). To improve dental health care, professional recommendations should always fit patients' specific needs (Silverman and Wilder, 2006). Given the strong adhesion of biofilms grown from whole saliva (Verkaik et al., 2010), a mechanical plaque removal strategy must be implemented to achieve satisfactory oral health. The introduction of a novel device may improve patients’ compliance (Chongcharoen et al., 2012; Sicilia et al., 2003). The aim of this study was to determine the biofilm elimination capability of a new oral care device, the digital brush (Enacare, Micerium S.p.A., Genoa, Italy), a disposable gauze product containing 0.12% chlorhexidine. This device can be used as an alternative to conventional oral hygiene, when performing the latter is difficult or as additional device to improve the quality of self-performed mechanical plaque removal. The null hypothesis of this study was that the presence of microbiota (representing the cleansing effect) before and after the use of a medicated gauze product on the mucosa and teeth would not differ.
MATERIALS AND METHODS
A disposable gauze product containing 0.12% chlorhexidine can serve as an alternative device for oral hygiene, even outdoors, or as an additional device for individuals with special care needs, bedridden patients, and caregivers.
Patients
The study group comprised 30 Caucasian patients (14 males and 16 females) with a mean age of 48.3 (range, 8 to 90) years. All patients provided written informed consent.
Sampling
At baseline, pre-treatment (pre-T) supragingival plaque samples were taken from the right vestibular and lingual mucosa in 15 subjects (group 1) and from the buccal aspect of the anterior sextant in 15 subjects (group 2) using sterile swabs. The subjects were instructed in proper oral hygiene and the use of the digital brush as a cleansing device (Figures 1 and 2), using a rolling motion technique (Figure 3A and B) for ~2 min. Post-treatment (post-T) microbiological sampling was performed immediately after cleaning.
Culture protocol
Saliva samples were collected with flocked swabs (Copan Italia S.p.A., Bresica, Italy) designed for biological sample collection that contained a transport medium specific to aerobic and anaerobic bacteria. Samples subjected to delayed (>24 h after collection) microbiological evaluation were transferred to cryovials and stored at –80°C to ensure preservation. Bacterial culture was performed as follows. Using a disposable sterile loop, 10-μl samples were streaked onto the following plates (Vacutest; Kima (ARZERGRANDE, Pd, Italy): horse blood agar (for non-selective growth of streptococci groups A to C, pneumococci, and staphylococci), azide agar (for selective growth and isolation of streptococci, including Enterococcus species), Herellea agar (for selective growth and isolation of Gram-negative bacteria), and CHROMagar Candida (for Candida identification). The plates were incubated at 37°C for 24 h, then examined to distinguish colonies on the basis of morphology, pigmentation, and macroscopic shape. In cases of positive growth, standard identification procedures were applied to selected colonies. The isolated colonies were identified using the VITEK® automatic system ((bioMérieux, Inc, Hazelwood, Mo). For colony counts, samples were serially diluted to 1:106. The number of colony-forming units (CFUs)/ml in the original sample was determined by multiplying the number of colonies (30 to 300) per plate by the dilution factor.
MATERIALS AND METHODS
A disposable gauze product containing 0.12% chlorhexidine can serve as an alternative device for oral hygiene, even outdoors, or as an additional device for individuals with special care needs, bedridden patients, and caregivers.
Patients
The study group comprised 30 Caucasian patients (14 males and 16 females) with a mean age of 48.3 (range, 8 to 90) years. All patients provided written informed consent.
Sampling
At baseline, pre-treatment (pre-T) supragingival plaque samples were taken from the right vestibular and lingual mucosa in 15 subjects (group 1) and from the buccal aspect of the anterior sextant in 15 subjects (group 2) using sterile swabs. The subjects were instructed in proper oral hygiene and the use of the digital brush as a cleansing device (Figures 1 and 2), using a rolling motion technique (Figure 3A and B) for ~2 min. Post-treatment (post-T) microbiological sampling was performed immediately after cleaning.
Culture protocol
Saliva samples were collected with flocked swabs (Copan Italia S.p.A., Bresica, Italy) designed for biological sample collection that contained a transport medium specific to aerobic and anaerobic bacteria. Samples subjected to delayed (>24 h after collection) microbiological evaluation were transferred to cryovials and stored at –80°C to ensure preservation. Bacterial culture was performed as follows. Using a disposable sterile loop, 10-μl samples were streaked onto the following plates (Vacutest; Kima (ARZERGRANDE, Pd, Italy): horse blood agar (for non-selective growth of streptococci groups A to C, pneumococci, and staphylococci), azide agar (for selective growth and isolation of streptococci, including Enterococcus species), Herellea agar (for selective growth and isolation of Gram-negative bacteria), and CHROMagar Candida (for Candida identification). The plates were incubated at 37°C for 24 h, then examined to distinguish colonies on the basis of morphology, pigmentation, and macroscopic shape. In cases of positive growth, standard identification procedures were applied to selected colonies. The isolated colonies were identified using the VITEK® automatic system ((bioMérieux, Inc, Hazelwood, Mo). For colony counts, samples were serially diluted to 1:106. The number of colony-forming units (CFUs)/ml in the original sample was determined by multiplying the number of colonies (30 to 300) per plate by the dilution factor.
The presence or absence of microorganisms (including Candida species, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus viridans, Enterobacter species) was determined before and after cleaning with the digital brush. Pearson’s chi-squared test was used to analyze bacterial concentrations and compare data from groups 1 and 2.
RESULTS The results of microbiological evaluation are reported in Table 1. The following bacteria were detected: Candida albicans (8 pre-T and 3 post-T), Candida spp. (3 pre-T and 0 post-T), Enterobacter spp. (2 pre-T and 2 post-T), S. aureus (12 pre-T and 4 post-T), S. epidermidis (2 pre-T and 1 post-T), Staphylococcus species (29 pre-T and 22 post-T), and S. viridans (29 pre-T and 22 post-T). Microbiota differed between sampling sites. No significant difference in the presence of bacteria was detected between groups 1 and 2. The mean post-T reduction in bacterial concentration was 2.36 log10. Using this cut-off value, data from groups 1 and 2 were compared by Pearson’s chi-squared test. Although more reduction was visible in group 2 samples (from the buccal aspect of the anterior sextant), no significant difference was found.
RESULTS The results of microbiological evaluation are reported in Table 1. The following bacteria were detected: Candida albicans (8 pre-T and 3 post-T), Candida spp. (3 pre-T and 0 post-T), Enterobacter spp. (2 pre-T and 2 post-T), S. aureus (12 pre-T and 4 post-T), S. epidermidis (2 pre-T and 1 post-T), Staphylococcus species (29 pre-T and 22 post-T), and S. viridans (29 pre-T and 22 post-T). Microbiota differed between sampling sites. No significant difference in the presence of bacteria was detected between groups 1 and 2. The mean post-T reduction in bacterial concentration was 2.36 log10. Using this cut-off value, data from groups 1 and 2 were compared by Pearson’s chi-squared test. Although more reduction was visible in group 2 samples (from the buccal aspect of the anterior sextant), no significant difference was found.
DISCUSSION
Studies of the oral microbial environment have demonstrated that oral mucosal tissues act as reservoirs of the bacteria that colonize tooth surfaces (Silverman and Wilder, 2006; Verkaik et al., 2010; Needleman et al., 2005; Gjermo and Flotra, 1970; Claydon, 2008). This finding supports the incorporation of an effective antimicrobial mouth rinse into the daily oral hygiene regimen to complement mechanical plaque control (Silverman and Wilder, 2006; Verkaik et al., 2010; Yuen et al., 2009; West and Moran, 2008; Gunsolley, 2006).

 Figure 3. The digital brush is wrapped around the index finger and utilized with a sweeping motion in an apico-occlusal direction from the oral mucosa to the tooth surfaces, similar to the roll brushing technique. Finger tactile receptors can guide cleaning movements to better reach frequently neglected dental surfaces in the posterior lingual/palatal areas. This device may improve the thoroughness and efficiency of cleaning.
Figure 3. The digital brush is wrapped around the index finger and utilized with a sweeping motion in an apico-occlusal direction from the oral mucosa to the tooth surfaces, similar to the roll brushing technique. Finger tactile receptors can guide cleaning movements to better reach frequently neglected dental surfaces in the posterior lingual/palatal areas. This device may improve the thoroughness and efficiency of cleaning.
In the present study, a greater reduction in microbial concentration occurred in group 2 (samples taken from the buccal aspect of the anterior sextant) than in group 1 (samples taken from the right vestibular and lingual mucosa), although this difference was not significant. Mechanical cleansing with the digital brush tended to be more effective on hard surfaces than on the mucous membranes. The lack of significant findings may be due to the small sample size. Further research may support our findings by detecting significant differences.
Within the limits of this clinical and microbiological evaluation of a small sample, the digital brush seems to be an effective plaque removal device. Its use as an alternative tool when conventional oral hygiene is difficult to implement or as a supplementary device to improve the quality of self-performed mechanical plaque removal can be recommended (Lucchese et al., 2012b). Further studies with larger samples are necessary to more fully evaluate the cleansing effectiveness of this novel device.
Bagis B, Baltacioglu E, Özcan M, Ustaomer S (2011). Evaluation of chlorhexidine gluconate mouthrinse-induced staining using a digital colorimeter: An in vivo study. Quintessence Int. 42(3):213-223.
Bopp M, Darby M, Loftin KC, Broscious S (2006). Effects of daily oral care with 0.12% chlorhexidine gluconate and a standard oral care protocol on the development of nosocomial pneumonia in intubated patients: A pilot study. J. Dent. Hyg. 80(3):9.
Claydon NC (2008). Current concepts in toothbrushing and interdental cleaning. Periodontol. 2000. 48:10-22.
Cuesta AI, Jewtuchowicz V, Brusca MI, Nastri ML, Rosa AC (2010). Prevalence of Staphylococcus spp. and Candida spp. in the oral cavity and periodontal pockets of periodontal disease patients. Acta Odontol. Latinoam. 23(1):20-26.
Gunsolley JC (2006). A meta-analysis of six-month studies of antiplaque and antigingivitis agents. J. Am. Dent. Assoc. 137(12):1649-1657.
Lucchese A, Bertacci A, Chersoni S, Portelli M (2012a). Primary enamel permeability: A SEM evaluation in vivo. Eur. J. Paediatr. Dent. 13:231-235.
Needleman I, Suvan J, Moles DR, Pimlott J (2005). A systematic review of professional mechanical plaque removal for prevention of periodontal diseases. J. Clin. Periodontol. 32 Suppl 6:229-282.repliche orologi
Sicilia A, Arregui I, Gallego M, Cabezas B, Cuesta S (2003). Home oral hygiene revisited. Options and evidence, Oral Health Prev. Dent. 1 Suppl 1:407-422.replica panerai
van der Weijden GA, Hioe KP (2005). A systematic review of the effectiveness of self-performed mechanical plaque removal in adults with gingivitis using a manual toothbrush. J. Clin. Periodontol. 32 Suppl 6:214-228.
West NX, Moran JM (2008). Home-use preventive and therapeutic oral products. Periodontol. 2000 48:7-9.
<div style="\\\\"text-align:" justify;\\\\"=""> Yuen HK, Tress ME, Salinas CF, Slate EH (2009). Effectiveness of oral self-care among adult Gullah-speaking African Americans with diabetes. Spec. Care Dentist. 29(3):128-133.replica watches(FONTE: ACADEMIC JOURNAL)
Torna indietro

